Reproducibility in Human Neuroimaging Research: A Practical Example from the Analysis of Diffusion MRI
Ariel Rokem
My name is Ariel Rokem. I am a Data Scientist at the University of Washington eScience Institute. My research training and experience have been mostly in the field of human cognitive neuroscience. During my postdoctoral training (2011-2015) in Prof. Brian Wandell's group, at the Department of Psychology at Stanford University, I conducted studies of human brain structure and function, using quantitative MRI. A focus of the research program that I started in Brian's lab is the application of ideas from statistical learning theory to measurements of human white matter with diffusion MRI (dMRI).
Workflow
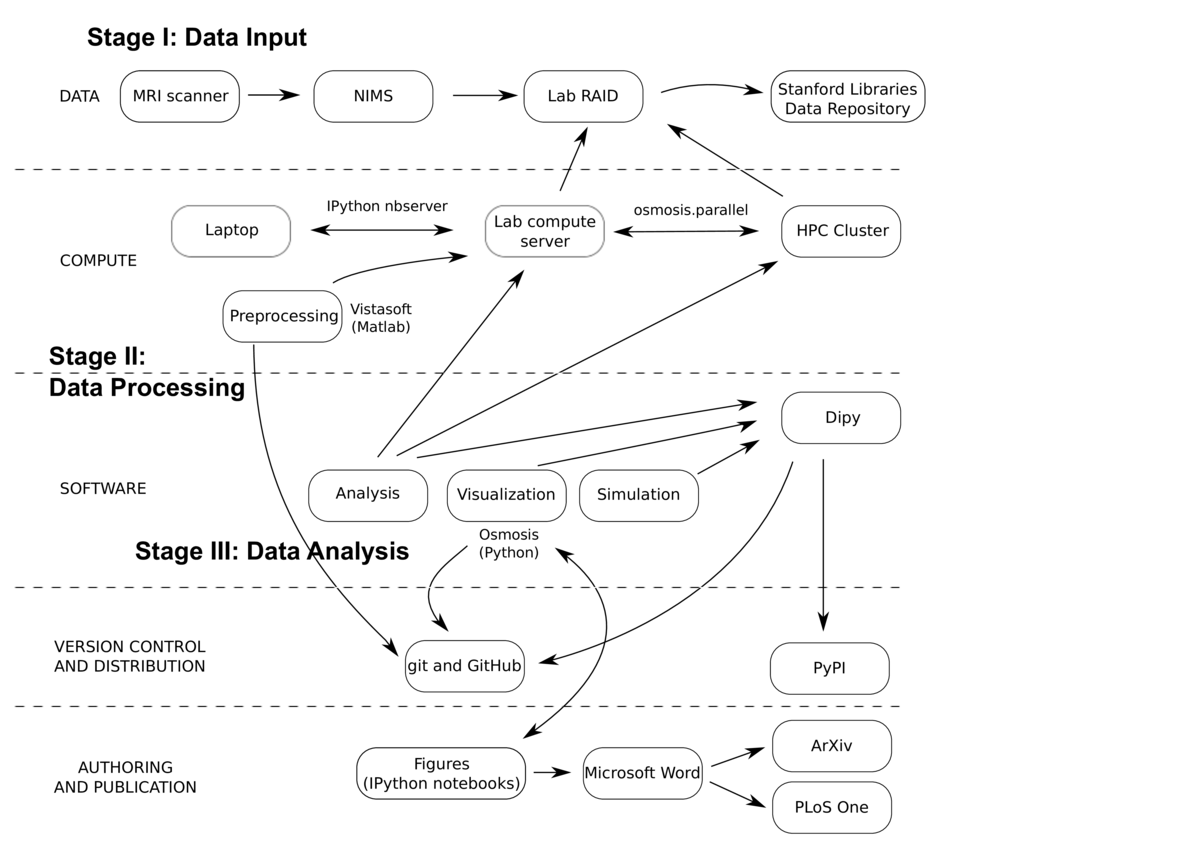 Measurements of dMRI are used as a way to assess the structure of the human brain and its connectivity in vivo. Many parameters of the measurement are determined by the experimenter, and incur trade-offs between sensitivity and signal-to-noise ratio (SNR). Models of the white matter based on different measurements are commonly used to make inferences about connectivity and tissue properties, but there was no extensive study of the fits of these models to the data, and no assessment of the effects of measurement parameters on the model fits. In the study described here, we used cross-validation to evaluate two commonly-used models in a variety of measurement conditions. The work was published in PLoS One
Measurements of dMRI are used as a way to assess the structure of the human brain and its connectivity in vivo. Many parameters of the measurement are determined by the experimenter, and incur trade-offs between sensitivity and signal-to-noise ratio (SNR). Models of the white matter based on different measurements are commonly used to make inferences about connectivity and tissue properties, but there was no extensive study of the fits of these models to the data, and no assessment of the effects of measurement parameters on the model fits. In the study described here, we used cross-validation to evaluate two commonly-used models in a variety of measurement conditions. The work was published in PLoS One
The project started with the collection of MRI data. Six participants were scanned in different experimental conditions. The data were collected in the Stanford Center for Neurobiological Imaging (CNI). The CNI has developed the Neurobiological Image Management System (Wandell, Rokem, Perry, Schaefer, & Dougherty, 2015), which captures the data from the scanner, archives it, and exposes a web interface that allows researchers to control access to the data, and copy it into the lab's data storage, a RAID (redundant array of independent disks) system.
The data were preprocessed using standard procedures (in the sense that any practitioner of MRI would perform these steps on his or her data). This includes correction of motion artifacts, alignment to a common coordinate frame, and tissue type segmentation. These steps were performed once, at the beginning of the study. The code that performs these steps is part of the lab code distribution, vistasoft, freely available through GitHub. Preprocessing also relied on freely available software from other labs.
These preprocessed data are publicly available through the Stanford Libraries Stanford Digital Repository (SDR), as two different collections: http://purl.stanford.edu/ng782rw8378 and http://purl.stanford.edu/rt034xr8593. Most of the data was licensed under the Creative Commons Atttribution license, and a small subset was also released under the Public Domain Dedication License, for unencumbered use in methods development.
Subsequent analysis was conducted on these preprocessed data, using a Python library: osmosis. This includes implementations of methods for fitting the data, statistical analysis, simulations and visualization, as well as utility functions to handle parallel execution on an HPC (high-performance computing) cluster. The library depends on many components of the scipy stack, including numpy, scipy, matplotlib, scikit-learn. In addition, the code depends on components of the neuroimaging in Python libraries. Approximately 30% of the module code was covered by unit tests, with a particular emphasis on core modules and utility functions that were reused. A few end-to-end tests were implemented to track regressions. Development of the software was done openly on GitHub, and it was also released under an attribution license.
Scripts using the module code were developed using the IPython notebook. These scripts were run and edited many times, and as the project evolved a few of these were copied into a documentation folder, with notebooks named "Figure1.ipynb", "Figure2.ipynb", etc, each corresponding to a figure in the paper. In writing the paper, these figures were saved and additionally manually edited by hand to add labels and annotation, and then integrated into a Word document file, which was used to collaborate on writing with the other authors. The writing process was not versioned throughout, but several versions of the article were submitted to the arXiv preprint server, while the article underwent peer review.
Most of the computations during the development of the project were conducted on a lab multi-core compute server that was running an IPython notebook server. Thus, much of the development of the code was done on a laptop, over a web browser, connected to the server. Some procedures described in the paper would require an inordinate amount of time without the access that we had to an HPC cluster. For example, testing different settings of model regularization parameters required fitting the models hundreds of times. Data was accesible to the cluster through a mount of the lab RAID. Tasks run on the cluster were managed through a queue system (Sun Grid Engine), and a module was developed (osmosis.parallel) to facilitate submission of code to the cluster. These scripts could not be used as IPython ipynb files, and were separately invoked on the command line, but they are included as part of the code distribution, recording these steps.
The IPython notebook documenting the steps that required parallel execution includes both a 'precomputed' version (where parameters of the analysis are read from precomputed files), and 'complete' versions, which include the code that would have to be run to reproduce these results entirely on a single machine. Precomputed parameter files were not made publicly available, and would have to be recomputed to reproduce the results in these notebooks.
Though reproduction of the results in the paper could, in principle, be achieved using this library, it is not neccesarily useful as a tool for others to work with, and not easy to extend beyond the models that we tested. During the work on this project, I became involved in an open-source project, which develops Python software for the analysis of dMRI data: Dipy. The main ideas in osmosis were eventually ported into Dipy, accomodating the application programming interface (API), documentation and testing requirements of that project. Furthermore, the prediction and cross-validation API that I implemented in Dipy is designed to be sufficiently general to accomodate new models, and mechanisms to evaluate their performance in fitting dMRI data.
Through Dipy, the code in this project is now also distributed widely through both GitHub and the Python Package Index (PYPI), under the permissive BSD license.
Pain points
One of the main difficulties encountered was the duration of some of the calculations. Some of the models, when fit on the entire brain volume, would require many hours. In particular, using the IPython notebook as a computational environment proved to be limiting, because connection to the kernel is only reliably maintained as long as the computer running the browser is kept on and prevented from sleeping. This also made it hard to perform computations that required a long duration in the notebook. One of the ways to deal with this was the development of caching mechanisms for model fit parameters. The models would be fit using a script, and the paramters cached to file. The model instantiation in the notebook would then know how to load these parameters from file, if the file existed.
Another point of frustration was that as the code in the modules evolved, code that was stored in the notebooks become outdated, and was no longer usable. This meant that as the analysis code itself evolved, new notebooks had to be written. Furthermore, as the writing and review of the article proceeded, figures were moved around in the article, and other figures got added; Figures that had started as appendices were integrated into the body of the article, etc. Thus, it might have been better to wait until the end result was an accepted article, and only then organize the entiree reproducible workflow that led to this result.
Key benefits
Though sometimes cumbersome and effortful, one of the major benefits of the process of producing a reproducible workflow is the level of confidence in the results. There is never a doubt about what code is associated with what result, because the full chain of evidence is documented in the code leading to that result.
Key tools
A specific module (osmosis.parallel) was developed to deal with submission of jobs to parallel execution on the HPC cluster. This module would read in a 'template' script, and then create from this template, Python script files that contained the instructions to run the fitting process with different conditions, or on different parts of the same brain. The creation of this module resulted in a highly reproducible process. Consequently reuse of elements of this module produced benefits in time-saving during the development of the analysis methods.
Questions
What does "reproducibility" mean to you?
Reproducibility is a matter of degree, not of kind. It usually depends on the availability of code and data from a scientific study, such that only a reasonable effort would be required to generate the evidence (numbers and visuals) used to support a scientific finding.
Ideally, a small number of commands at the command line would suffice, but in some complex cases, more work could be required. A reasonable amount of effort required might be rather extensive, when large amounts of data storage, or large amounts of computation are needed.
A higher standard, sometimes called 'replicability' would be to require that the same conclusions be reached if another group of researchers were to do the same experiments, and implement the same ideas in their analysis.
Reproducibility does not guarantee replicability (Leek & Peng, 2015). Some may even argue that reproducibility and replicability may sometimes be in conflict, because implementation errors can be propagated in reproduction, but not in replication (Baggerly, Morris, Edmonson, & Coombes, 2005; R. D. Peng, 2009).
Why do you think that reproducibility in your domain is important?
Human neuroscience is a field which is particularly likely to have an abundance of false findings (Ioannidis, 2005): Sample sizes are usually small, particularly in MRI, which is an expensive experimental technique. The standards of the field focus on statistical significance of effects, rather than effect sizes, which tend to be small. Though standards limiting the selection of tested relationships, and limiting the flexibility of experimental and analytic designs are starting to emerge, in practice these are not very strictly limited. Some of the aspects of the field that make it interesting and important, are also pernicious in this regard: the direct application to human health means that there is a perception of potential financial incentives. Finally, it is a burgeoning field, with many groups working on similar questions. Higher standards of reproducibility in this case would mean less false findings, because at least some of these factors would be ameliorated by a full "chain of evidence" to support every finding.
How or where did you learn about reproducibility?
Many of these practices evolved out of laziness. Early on in grad school, I learned that most analyses that are done once eventually need to be redone, and that ultimately I would have to do less work, not more, if I had a script that generated all my figures for every study that I was doing. This also evolved from being rather bad at taking notes about the work I was doing in the lab. I would need programs, and eventually IPython notebooks, just to remember what I did to get from the data to the conclusions.
A huge impact was the mentorship I got from Fernando Perez during graduate school. He was not shy about how little of the research in our field he believed to be true, and this skepticism inspired me to struggle to be more confident in my own research conclusions.
What do you see as the major challenges to doing reproducible research in your domain, and do you have any suggestions?
There are several barriers to wider adoption of reproducible research practices in human neuroscience. The first is that there is very little practical cost to not being reproducible. As mentioned above, there is likely to be a large proportion of false results in the neuroscience literature, and it's more likely to be false if it's not reproducible. Since a false positive result is more likely to result in a publishable unit, there seem to be incentives in place to not be reproducible, slowing down the progress of the entire field.
What do you view as the major incentives for doing reproducible research?
The level of confidence that I have in my results is quite high. That helps me sleep well at night.
Would you recommend any specific resources for learning more about reproducibility?
There are several papers that provide guidelines for reproducibility with a specific focus on neuroimaging. Two recent examples include Gorgoloewksi & Poldrack (2016) and Pernet & Poline (2015).
References
Baggerly, K. A., Morris, J. S., Edmonson, S. R., & Coombes, K. R. (2005). Signal in Noise: Evaluating Reported Reproducibility of Serum Proteomic Tests for Ovarian Cancer. J Natl Cancer Inst, 97, 307–309.
Gorgoloewksi, K., & Poldrack, R. (2016). A practical guide for improving transparency and reproducibility in neuroimaging research. J Natl Cancer Inst, 14(7), e1002506. http://doi.org/http://dx.doi.org/10.1371/journal.pbio.1002506
Ioannidis, J. P. A. (2005). Why Most Published Research Findings Are False. PLoS Med, 2(8), e124. http://doi.org/10.1371/journal.pmed.0020124
Leek, J. T., & Peng, R. D. (2015). Opinion: Reproducible research can still be wrong: Adopting a prevention approach. Proceedings of the National Academy of Sciences, 112(6), 1645–1646. http://doi.org/10.1073/pnas.1421412111
Peng, R. D. (2009). Reproducible research and Biostatistics. Biostatistics, 10, 405–408.
Pernet, C., & Poline, J. B. (2015). Improving functional magnetic resonance imaging reproducibility. Gigascience, 4(15). http://doi.org/http://dx.doi.org/10.1186/s13742-015-0055-8
Wandell, B. A., Rokem, A., Perry, L. M., Schaefer, G., & Dougherty, R. F. (2015). Data management to support reproducible research. arXiv, 1502.06900v1.